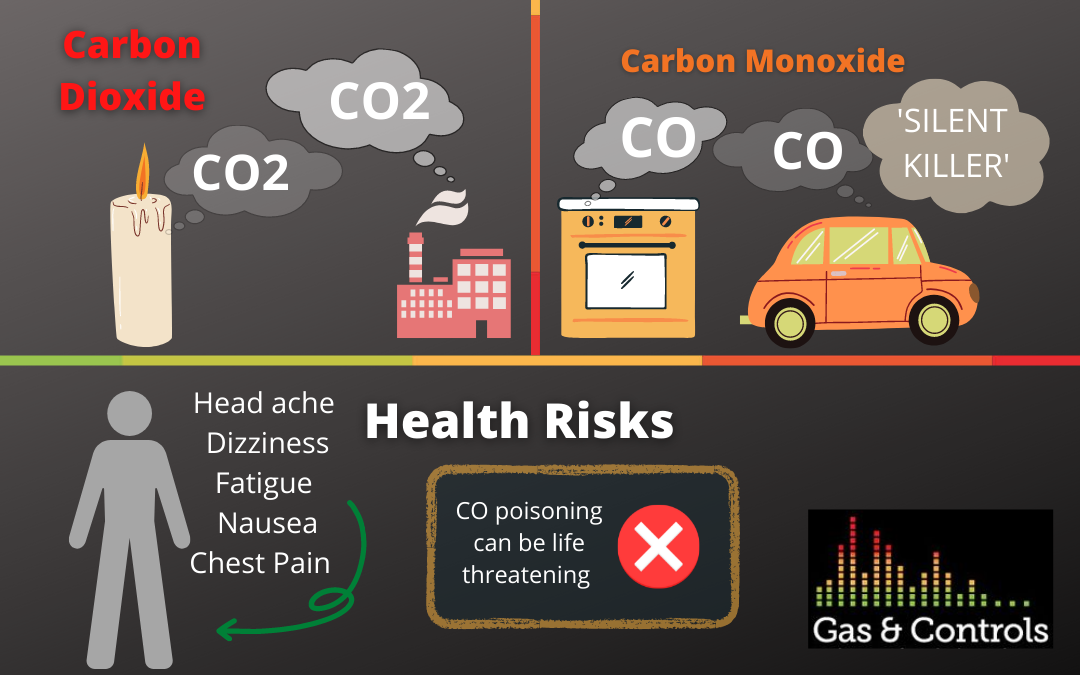
Many people confuse carbon monoxide and carbon dioxide. Let’s explore a basic explanation of each gas before understanding where each gas affects people, and the environment and how to test for each. Both gases have similar names as they are both a combination of carbon and oxygen, however, they are created through different chemical reactions.
Where does Carbon Dioxide come from?
Complete combustion is a chemical reaction where hydrogen reacts with oxygen producing carbon dioxide and water. Carbon dioxide is a result of this process. Complete combustion often involves a flame, similar to watching a candle burn. Candle wax is a hydrocarbon, which reacts with oxygen in the air and the heat from the lit wick, releasing the carbon dioxide into the air; complete combustion. The carbon dioxide is a colourless, odourless gas. It quickly mixes through the atmosphere as it is a largely non-reactive gas.
Industrial plants which produce hydrogen or ammonia from natural gas, coal or large-volume fermentation operations are amongst the largest commercial producers of carbon dioxide. As well as industrial process, carbon dioxide also has many applications within the food and beverage industry.
Where does Carbon Monoxide come from?
Alternatively carbon monoxide is a result of incomplete combustion. Incomplete combustion happens when there is a limited supply of air, so only half as much oxygen adds to the carbon which forms carbon monoxide. (CO= one oxygen atom, CO2= two oxygen atoms).
Carbon Monoxide, unlike Carbon Dioxide does not occur naturally in the atmosphere. Carbon Monoxide is created through the incomplete combustion of coal, natural gas, and oil. Low levels of oxygen and low temperatures lead to carbon monoxide in the combustion mixture.
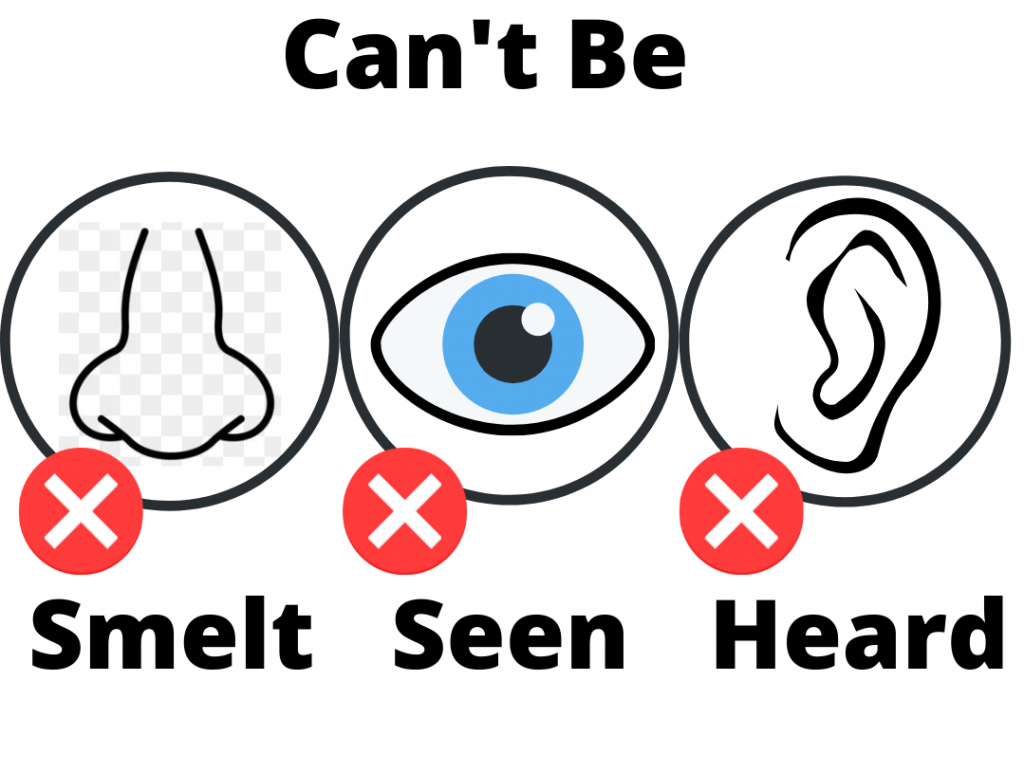
Fuel burning appliances, including gas furnaces, gas stoves, gas dryers, gas water heaters, fireplaces and cars produce dangerous levels of carbon monoxide. A gas detector must be used to identify carbon monoxide as it is a colourless, odourless gas.
The internal combustion engine is the main source of carbon monoxide within industrial settings. Workers in areas of confined or enclosed spaces such as garages, tunnels, loading docks, manholes, vehicle repair shops and warehouse are also at high risk. Large amounts of Carbon Monoxide are produced from furnaces and ovens, especially when not maintained properly. Workers working near trucks, forklifts and other type of equipment are also at higher risk.
Carbon Monoxide is typically an unwanted by-product, however many industries such as metal fabrication, chemical manufacturing and pharmaceuticals package carbon monoxide. Carbon Monoxide is also used in electronic and semiconductor applications, and for reducing ores when manufacturing metal carbonyls.
Health Risks for Carbon Monoxide and Carbon Dioxide
High concentration in a confined space can be toxic, however carbon dioxide is poisoning is rare. Carbon Dioxide creates an environment for asphyxiation through using up the space in the air instead of oxygen. At low concentration levels of less than 30,000 PPM mild carbon dioxide poisoning can cause headaches, dizziness, fatigue and nausea. Carbon Dioxide can become life-threatening at 80,000 ppm. CO2 has a permissible exposure limit (PEL) of 5.000 PPM over an eight hour time period and 30,000 PPM over a 10-minute time period, as set by Occupational Safety and Health Administration (OSHA).
Carbon Monoxide, often referred to as the ‘Silent Killer’ is far more dangerous. CO is a colourless, odourless, tasteless, non-irritable gas, deeming early signs of poisoning extremely difficult to detect. Carbon Monoxide binds to the parts of your blood which carries oxygen molecules, chemically blocking your body and organs from getting oxygen it vitally needs. This is why Carbon Monoxide is so dangerous. Exposure levels considered immediately dangerous to life and health is 1500 PPM and OSHA identifies the permissible exposure limit as 50 PPM over an eight hour period.
Using Gas Detectors to Measure Carbon Dioxide
It is very important to remember when choosing an industrial gas detector for the workplace that a single-gas carbon monoxide detector will not measure carbon dioxide, and vice versa. The sensors within each single gas detector are specific to each gas. There are multiple options when choosing the best gas detector for monitoring both carbon monoxide and/ or carbon dioxide, including personal single-gas monitors, multi-gas monitors and area monitors. When choosing the correct instrument it is vital the environment and properties of the gas or gases going to be monitored are explored.